Biophysical Projects
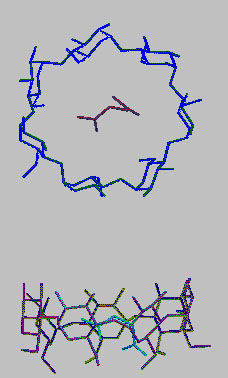
Crystal and molecular structure of β-cyclodextrin
inclusion complex with succinic acid
inclusion complex with succinic acid
(with Yu. V. LISNYAK - Department of Molecular Modeling, I. Mechnikov Institute of Microbiology and Immunology AMS of Ukraine, Kharkov 61057, Ukraine; V. N. BAUMER & O.V. SHISHKIN - STC ‘‘Institute for Single Crystals’’ NAS of Ukraine, Kharkov 61001, Ukraine)
The crystal complex of b-cyclodextrin with succinic acid, intermediate product of hydrolysis reaction of succinic anhydride in the presence of b-cyclodextrin, was isolated and studied by X-ray analysis (monoclinic, space group P21, a = 15.1977(7) Å, b = 10.1763(5) Å, c = 20.6943(6) Å, β = 109.239(4)°, V = 3021.8(2) Å3, Z = 2, R1= 0.0359, wR2= 0.0947). It was proved that β-cyclodextrin and succinic acid form an inclusion complex, which exists in crystal state as a heptahydrate. The molecule of succinic acid is fully included in the β-cyclodextrin cavity with its carboxyl groups accessible for water molecules. Water molecules located at borders of cavity rims and in interstices between molecules of β-cyclodextrin participate in formation of intermolecular hydrogen bonds. The overall structure does not contain disordered fragments. The crystal conformation of succinic acid corresponds to one of possible conformers of the molecule in vacuo and is almost not disturbed by intermolecular interactions in crystal. Based on the analysis of structural features of the crystal conformation of succinic acid and character of its location in the β-cyclodextrin cavity, it was suggested that hydrolysis of succinic anhydride via ring opening and formation of succinic acid is mediated by cyclodextrin microenvironment and it likely occurs near the narrow rim of the macrocycle cavity.
 |
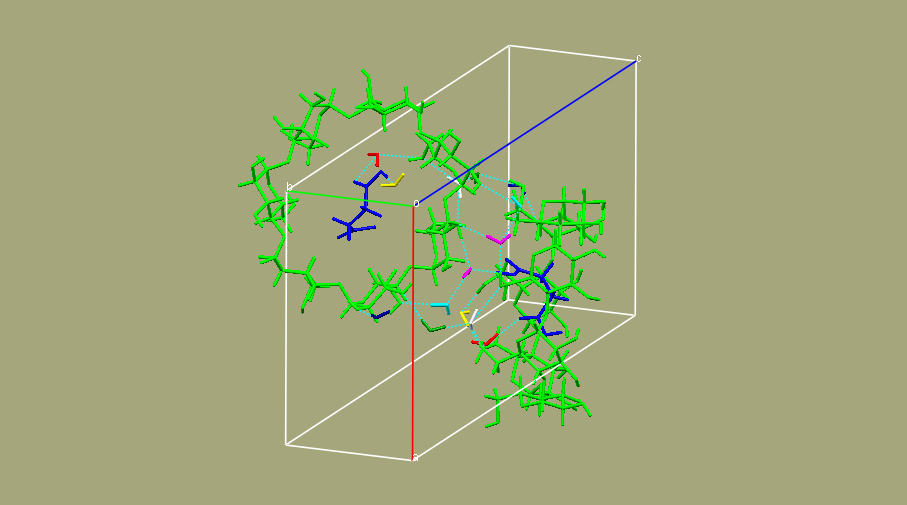 |
Structure and dynamics of water inside of KcsA ion channel (project in progress)
(with P.G. KUSALIK - Department of Chemistry, University of Calgary, AB Canada; D.F. WEAVER - Department of Chemistry, Dalhousie University, Halifax, NS Canada; A.P. LYUBARTSEV – Department of Physical, Inorganic & Structural Chemistry,
University of Stockholm, Sweden)
The ion permeation and the conformational state of the KscA channel have been the focus of theoreticians working in this field and noticeable progress in the understanding of these phenomena has already been achieved. Surprisingly few papers have been published where the hydration of the ion channel and the role of water in the transport of ions through its pore have been studied (e.g. L. Guidoni et al. FEBS Letters, 2000, 447, 37). The detailed structure and dynamics of water inside of KcsA ion channel is the primary focus of our investigation.We have followed examples from the previous literature (particularly, Chung, S.-H.; Kuyucak, S. Eur. Biphys. J., 2002, 31, 283) and developed a realistic model of KcsA, which is comprised from the channel forming protein imbedded into the lipid bilayer, bulk and “intrinsic” water and ions. In our analysis we will use the novel computational technique known as spatial distribution functions (SDFs). Relying on our previous experience in the investigation of hydration of amino- and hydroxy- groups in aqueous solutions of 1,2-disubstituted ethanes by means of SDF’s (A.V. Gubskaya and P.G. Kusalik, J. Phys. Chem. A, 2004, 108, 7151-7178), we will examine hydration patterns around selected polar groups on amino acid residues located in the cavity of KcsA. The hydrogen-bonding structure of “bulk” water inside the cavity will be compared with that from the hydration shell of the potassium ion and with that of a single water molecule located in the binding site of the filter.

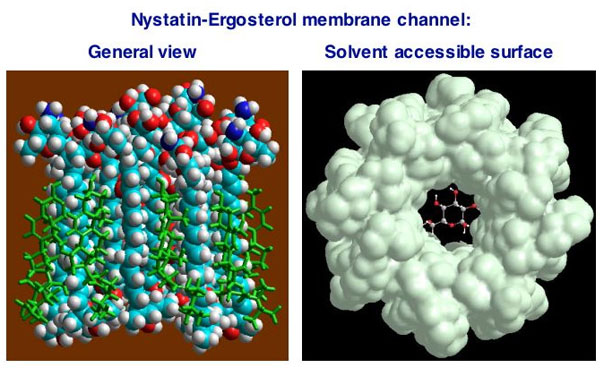
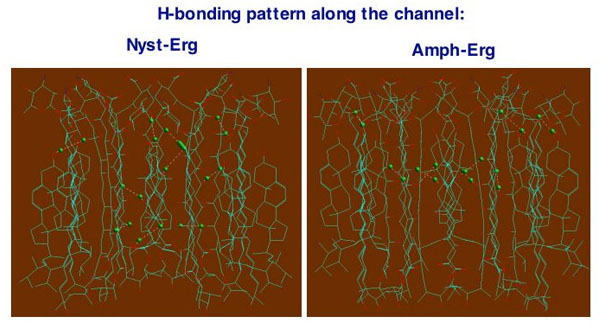
Molecular modeling study of polyene-sterol membrane channel
(with Yu. V. LISNYAK - Department of Molecular Modeling, I. Mechnikov Institute of Microbiology and Immunology AMS of Ukraine, Kharkov 61057, Ukraine)
Polyene macrolide antibiotics, usually represented by amphotericin B (Amph B) and nystatin (Nyst), are well known for several decades as membrane-acting agents that are widely used in medicine to treat advanced fungal infections. Polyene antibiotics (PA) exhibit a whole variety of valuable chemotherapeutical properties and despite of some side effects they are often considered as major antifungal drugs due to their high efficacy. Better understanding of molecular mechanisms responsible for PA functioning is of utmost importance for structure-aided design of new PA derivatives with reduced side effects. It has been recently hypothesized that molecular mechanisms of PA functioning are closely related to their ability to form hydrophilic channels in hydrophobic environment of a cellular membrane that causes leakage of ions and other small molecules from a cell (M. Baginski, J. Czub ,and K. Sternal, Chem. Rec., 2007,6, 320). Membrane sterols such as cholesterol and ergosterol also participate in formation of membrane channels as molecular targets for PA. Experimental molecular structure of polyene-sterol membrane channels (PSMC) has not been obtained yet and despite of the fact that PSMC were investigated in several experimental and theoretical studies (e.g., M. Gagos, R. Koper, and W.I. Gruszecki , Biochim. Biophys. Acta, 2001, 1511, 90) the structural specificity and characteristics of PSMC still remain subjects of discussion. To gain valuable insights into spatial organization of PSMC supramolecular aggregates, intermolecular interactions that involve their structural subunits and molecular mechanisms of antimicrobial function of membrane-acting agents we developed models of PSMC designed for both single-length and double-length channels. Intermolecular interactions (including hydrogen bonding) between antibiotic and sterol molecules, which are involved in isolated dimer as well as in supramolecular PSMC complex, are analyzed. Shape and dimensions of the channel and its pore, intermolecular hydrogen bonding pattern, contribution of different functional groups into hydrophilic and hydrophobic intermolecular interactions are discussed and compared with those for PSMC models available from the literature. The proposed model structures of PSMC can be easily incorporated into phospholipid bilayer of a cell membrane to serve as an initial structure for further comprehensive molecular dynamics simulations of this system in the presence of aqueous environment.